Purification of organic compounds:
Extraction: Removal of desired compound from a mixture
Alkaloids
Naturally occurring organic compounds with at least 1 nitrogen in them
,essential oils of flowers, leaves & vegetables, colouring matter of leaves and soluble constituents of some roots are extracted by heating with
suitable
Water,alcohol or benzene
solvent
Fluid in which things are dissolved
in Soxhlet apparatus
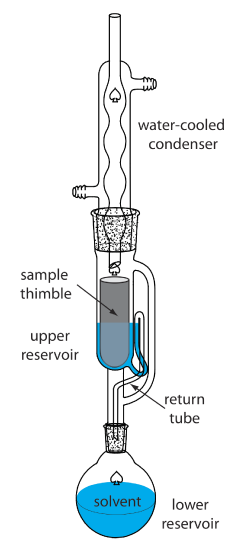
Purification:Process of increasing concentration of desired compound
SublimationDirect conversion of substance from solid phase to gas phase is used for sublimable compounds
CrystallisationFormation of crystals of a substance is used for Benzanoic acid
Simple distillationBoiling a mixture of substances with very different boiling points in a special setup in order to extract them is used for chloroform,aniline mixture
Fractional distillationDistillation performed with a fractionating column added to the setup for extraction of substances with similar boiling points from a mixture is used in petroleum industry
Distillation under reduced pressure is used in spentyle soap industry
Steam distillationDistillation in which steam is passed through steam volatile compound and compound is extracted from steam by condensing the steam and using a separating funnel is used for aniline water mixture
Sugars are separated using BAW in chromatographyMethod of extraction based on polarity difference.Compound is placed onto the stationary phase and the moving phase is passed over it and detected using aniline hydrogen phthalate
Amino acids are separated using either BAW or BEW in chromatography and detected using ninhydrin solution
Gas chromatographyChromatography where a mixture of gases and the moving phase passes through a long column coated by the stationary phase and a detector detects the time when gases exit along with their concentrations and records them onto a chart recorder can be used to separate amino acid mixtures in very low concentrations (up-to nanograms)
4:1:3 volume ratio of n-butanol,acetic acid,water is called BAW
6:4:3 volume ratio of n-butanal, ethanal, water is called BEW




